Welcome To The Medical Research Group
We are a clinical research organization that specializes in clinical trial management.
Our goal is to establish clinical research - collaborating with the industry as well as conducting scholarly research projects in the community. We are a physician-led, physician-driven, and physician-managed Institution
What we do
Protocol Writing and Review
Trial-related documents archival and maintenance
Patient informed consent form (ICF)
Timely submission for Institutional Review Board (IRB) approval
Contract negotiations with the clinical drug trials accountability and integrity
Compliance and Regulatory Merge
Advising & Alerting of potential protocol violations
Site initiation and trial close-out operational
Reporting serious adverse events to the sponsor or CRO and the IRB/IEC
Patient recruitment
Patient follow-up
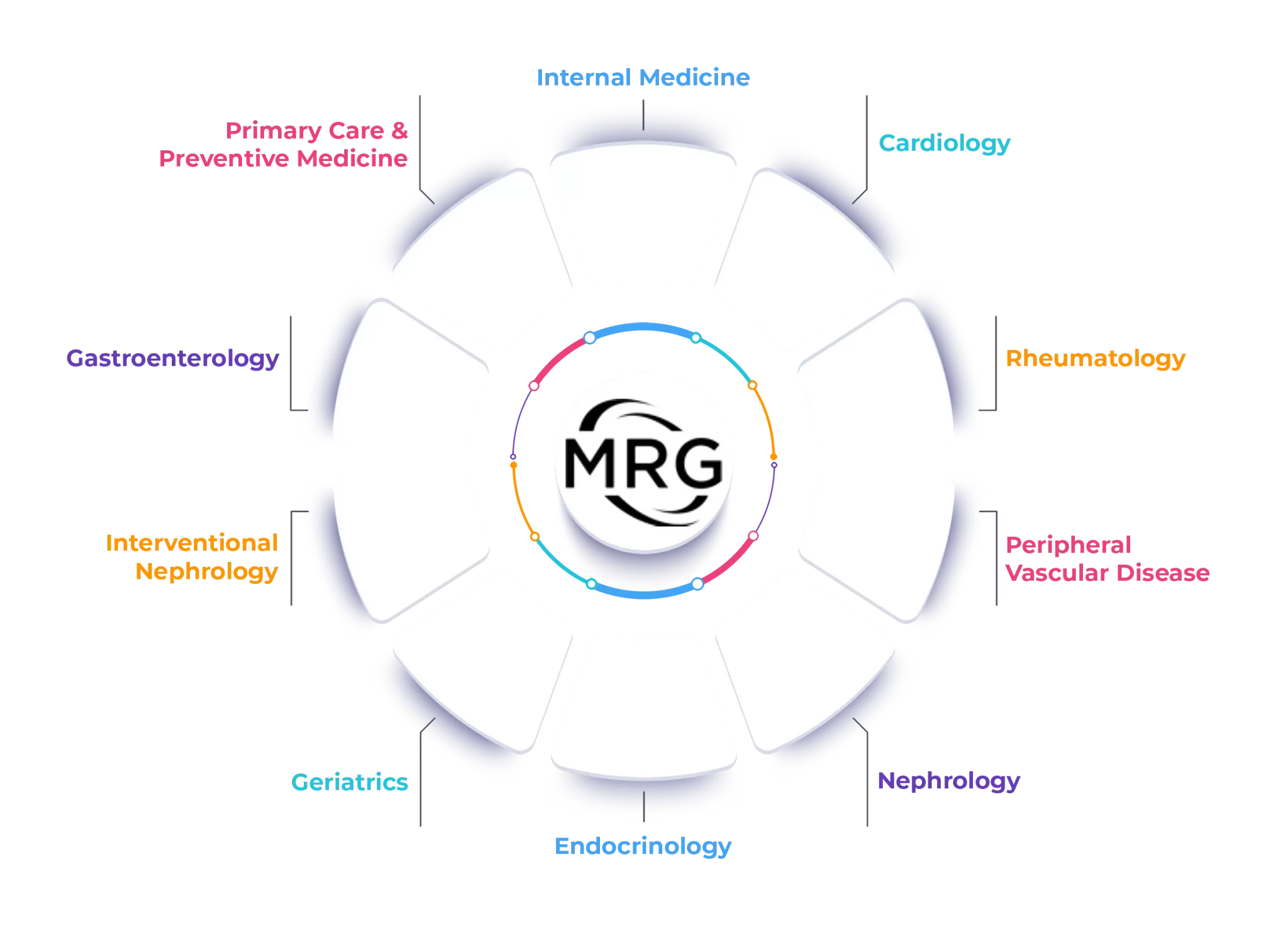
Areas of Interest